Abstract
Introduction
Clearance of infused factor VIII (FVIII) varies approximately 2-fold between persons with severe hemophilia A. This results in significant interpatient differences in factor levels following an infusion of FVIII and contributes to potentially significant differences in protection against spontaneous musculoskeletal bleeding in patients on fixed dose prophylaxis regimens.
Aim
The aim of this study is to compare two PK protocols: 1) a 6-point PK protocol with a 72 hour washout; and 2) a 2-point, one clinic visit PK protocol with no washout using the following pharmacokinetic (PK) parameters: clearance (Cl) and time to FVIII:C of 1% above baseline (tt1%) in persons with severe hemophilia A.
Methods
Inhibitor negative males with severe hemophilia A (FVIII<2%) receiving a standard half-life recombinant FVIII (rFVIII) concentrate (ADVATE®) were consented into a research ethics board approved study. In the 6-point PK protocol, participants were infused with approximately 50 IU/kg rFVIII after a minimum washout of 72 hours and FVIII levels were measured pre-infusion and at 1, 3, 9, 24 and 48 hours post-infusion. The 2-point PK protocol consisted of a blood sample taken in clinic approximately 24 hours after the participant infused their prophylactic dose at home (15-50 IU/kg), followed by a 25 IU/kg dose given in clinic and a 3 hour post-infusion sample. Frozen plasma samples were sent to a central laboratory in Kingston, Ontario where one-stage and chromogenic FVIII assays were performed. PK parameters (Cl and tt1%) were estimated using the 2 compartmental models of PK programs Phoenix WinNonlin 7.0 (Certara USA Inc.) and myPKFiT version 3.0 (Baxalta US Inc). Intra-class correlations (ICCs) were used to compare the PK parameters derived from the two PK protocols using WinNonlin and myPKFiT.
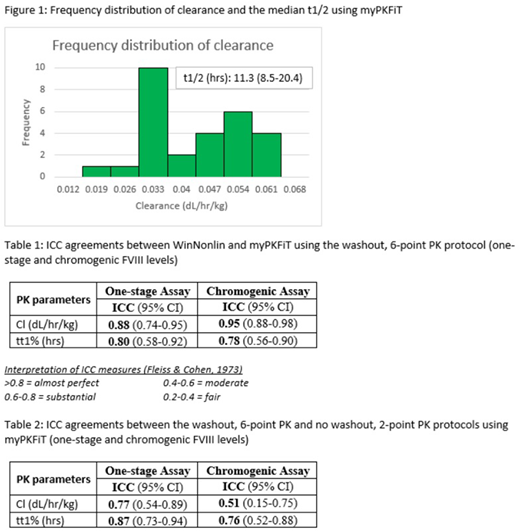
Results
28 males (median age: 12 years, range: 2-69 years) participated. The frequency distribution of clearance and the median half-life (t1/2) generated using myPKFiT is presented in Figure 1. There was a substantial to almost perfect agreement observed when comparing the PK parameters derived from the 6-point PK protocol with washout using the two PK programs (Table 1). There was a moderate to almost perfect agreement observed when comparing the PK parameters derived from the 6-point PK protocol with washout to the 2-point PK protocol with no washout using the myPKFiT program (Table 2).
Conclusion
The 2-point, one clinic visit, PK protocol (24 and 3 hrs) with no washout offers a convenient and practical approach to generating clinically relevant PK parameters in persons with severe hemophilia A. It can provide information relevant to selection of personalized prophylaxis regimens that aim to reduce to a minimum/eliminate spontaneous joint bleeding.
Blanchette:Shire: Other: Investigator-initiated research funding; Novo Nordisk: Other: Speaker's fees; Shire: Other: Speaker's fees; Bayer: Other: speaker's fees; Bioverativ: Other: Investigator-initiated research funding; Pfizer: Other: Speaker's fees. Jackson:Pfizer: Honoraria; Roche: Honoraria; Bayer: Honoraria; Novo Nordisk: Honoraria; Shire: Honoraria; Bioverativ: Other: Investigator initiated grant funding. Carcao:Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; LFB: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL-Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Khoo:Shire: Research Funding; Biogen Idec: Research Funding. Blatny:Shire, Pfizer, Roche: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.